Arexvy: First FDA-Approved RSV Vaccine for Older Adults
Respiratory Syncytial Virus (RSV) stands as a prevalent cause of respiratory illness, leading to approximately 177,000 hospitalizations and an estimated 14,000 deaths among adults aged 65 years and older annually in the US.
Following a winter marked by a surge in RSV-related hospitalizations, GSK’s Arexvy has emerged as a game-changer. On May 3, 2023, the FDA granted its long-awaited approval to Arexvy, the pioneering RSV vaccine specifically designed for adults aged 60 years and above.
By targeting RSV-related lower respiratory tract disease (LRTD), this groundbreaking vaccine promises to significantly reduce the burden of severe RSV infections. The decision to approve Arexvy was supported by compelling data from the successful pivotal AReSVi-006 phase III trial, showcasing exceptional efficacy even in older adults with underlying medical conditions and severe RSV disease. The vaccine is slated to be available in the United States ahead of the 2023/24 RSV season, heralding a new era in respiratory health protection.
GSK’s Arexvy Outshines Competition as First Approved Global RSV Vaccine for Older Adults Approved
In a significant milestone achieved on May 3, 2023, GSK Plc’s Arexvy gained its initial approval. This prophylactic vaccine utilizes immunostimulants to activate the immune system, providing crucial defense against Respiratory Syncytial Virus (RSV) infections.
RSV is a prevalent virus notorious for causing severe respiratory illnesses, particularly in young children and older adults. Arexvy’s primary objective is to prevent RSV infections, positioning it as a vital weapon in the ongoing battle against this ailment. With its inaugural approval, Arexvy presents healthcare providers with a fresh option to safeguard patients from this virus and mitigate potential complications that may arise.
Arexvy is the first and only approved vaccine worldwide for preventing RSV in older adults. The approval of it represents a research milestone after decades of unsuccessful efforts to develop an effective shot for the virus. It could be followed later this month by another RSV vaccine, from Pfizer, that’s under FDA review.
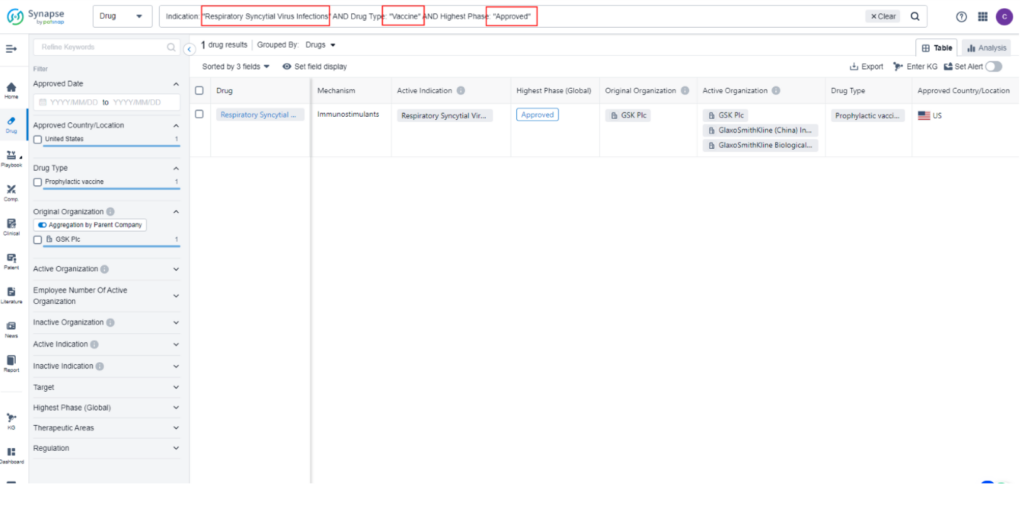
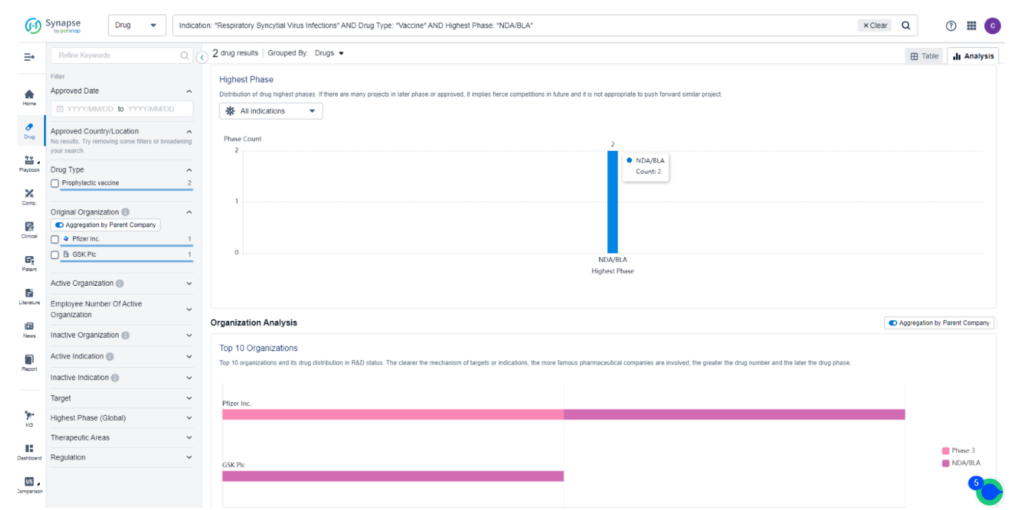
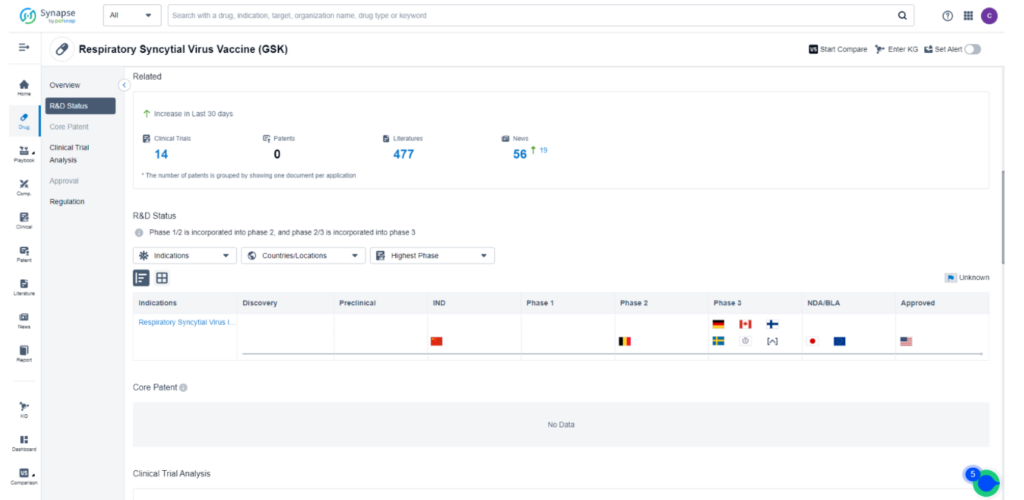
As a testament to its promising potential, Arexvy is currently the subject of 14 ongoing clinical trials worldwide, all dedicated to investigating its effectiveness in combating RSV Infections. While the drug has already obtained approval for sale in the United States, it is currently undergoing the New Drug Application/Biologics License Application (NDA/BLA) stage in Japan and the European Union. For detailed and comprehensive data regarding these impactful clinical trials, we encourage you to refer to Synapse, your go-to resource for the latest information in the field.
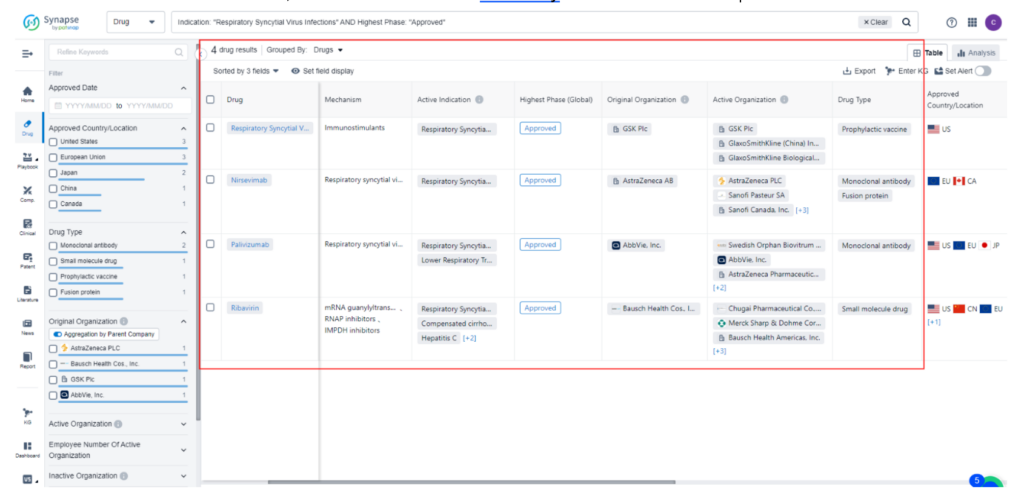
PatSnap Synapse reveals that the global landscape for RSC treatment consists of four approved drugs. Among these, two are classified as monoclonal antibodies, one falls under the category of small molecule chemistry, and the remaining one is a vaccine. This diverse range of approved treatment options highlights the ongoing efforts to combat RSC effectively on a global scale.
Tony Wood, Chief Scientific Officer, GSK, said: “The approval of Arexvy marks a turning point in our effort to reduce the significant burden of RSV. Arexvy is the first approved RSV vaccine for older adults, expanding GSK’s industry-leading vaccine portfolio, which protects millions of people from infectious diseases each year. Our focus now is to ensure eligible older adults in the US can access the vaccine as quickly as possible and to progress regulatory review in other countries.”
GSK predicts that its Arexvy vaccine will achieve sales figures in the “multi-billion” dollar range, akin to the success of its top-selling shingles vaccine, Shingrix. The company has taken proactive measures to ensure readiness, with millions of doses of Arexvy readily available for shipment.