August 2023 Global Innovative Drug Report
Our August 2023 drug report offers an in-depth look into the pharmaceutical industry’s recent innovations, diving deep into individual drug analyses. Within, you’ll find details on each drug’s profile, R&D status, active patents, mechanisms of action, clinical trials, competitive dynamics, and more.
This report consists of four parts:
- August new drug approvals
- New drug analysis
- Global drugs under Expedited Review Pathway
- Analysis of selected ERP drugs
Report Overview
1.) August Drug Approvals
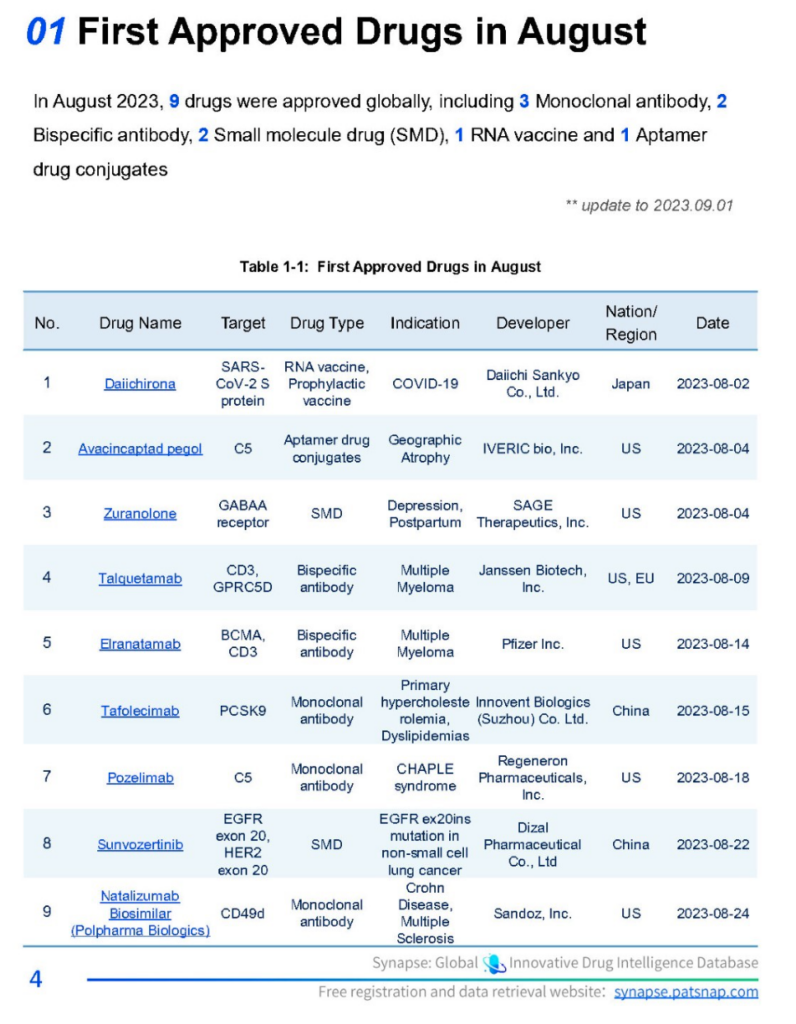
In August 2023, a total of nine drugs received global approval. This included three monoclonal antibodies, two bispecific antibodies, two small molecule drugs (SMDs), one RNA vaccine, and one aptamer drug conjugate.
2.) New Drug Analysis
This report features in-depth analyses of five newly approved drugs, including a thorough examination of Avacincaptad pegol.
Avacincaptad pegol treats the severe eye condition known as geographic atrophy. It aims to delay vision deterioration by preventing the immune system from damaging eye cells.



3.) Global Drugs Under Expedited Review Pathway
What’s more, 56 drugs were placed on ERP in August, including 35 orphan drug designations, nine with fast track status, four targeting rare pediatric diseases, three under breakthrough therapy designation, three with priority reviews, two receiving accelerated approvals, and one granted conditional marketing approval.

4.) Analysis of Selected ERP Drugs
This report also highlights five ERP drugs for detailed analysis. Focusing on Savolitinib — a receptor tyrosine kinase inhibitor targeting the mesenchymal-epithelial transition factor (MET), here’s what we uncovered:


Gain Access to the Comprehensive Report for FREE – Download Now! If you aren’t registered for Synapse (registration is required to download the report), click here to register for free.
Patsnap Synapse Database: An Overview and Key Features
Discover the innovative PatSnap Synapse database – an AI-powered platform that provides intuitive interfaces, curated content, and access to massive information sources. With integrated access to company data, diseases, targets, clinical studies, and biological and chemical entities, our platform offers a powerful search and association experience. Sign up for free today and experience the benefits for yourself!
R&D Decision Makers
Our mission is to empower R&D decision makers with swift access to accurate and connected data, facilitating their understanding of emerging technology trends, competitive landscapes, and partnership opportunities. By providing comprehensive insights, our platform helps decision makers to navigate and steer the direction of innovation with confidence.
Business Development Professionals
We offer a comprehensive database that covers the drug pipeline and investment history for over 320,000 life science organizations. This invaluable resource allows you to make informed decisions regarding potential partnerships or acquisition targets with confidence. Gain access to our extensive database and unlock the insights you need to drive your business forward.
Pharmaceutical Analyst
Our platform is designed to accelerate the research process for pharmaceutical analysts by leveraging a wealth of connected data, including drug approvals, clinical trials, patents, non-patent literature, and news. With Synapse, users gain a comprehensive 360-degree view of the competitive and technological landscape, empowering them to make informed decisions quickly and efficiently. Discover the power of Synapse and revolutionize your research today.
Copyright Statement: This report is the sole property of Patsnap and is protected under copyright laws. Any reproduction, excerpting, or other use of this report without explicit authorization from Patsnap is strictly prohibited. Authorized products must be used within the scope of authorization and must include a clear indication of the source. Patsnap reserves the right to investigate any violations of this statement and pursue legal action as necessary. For inquiries regarding authorization, please contact [email protected].
