FDA Approves Incyte’s Zynzy for Metastatic MCC
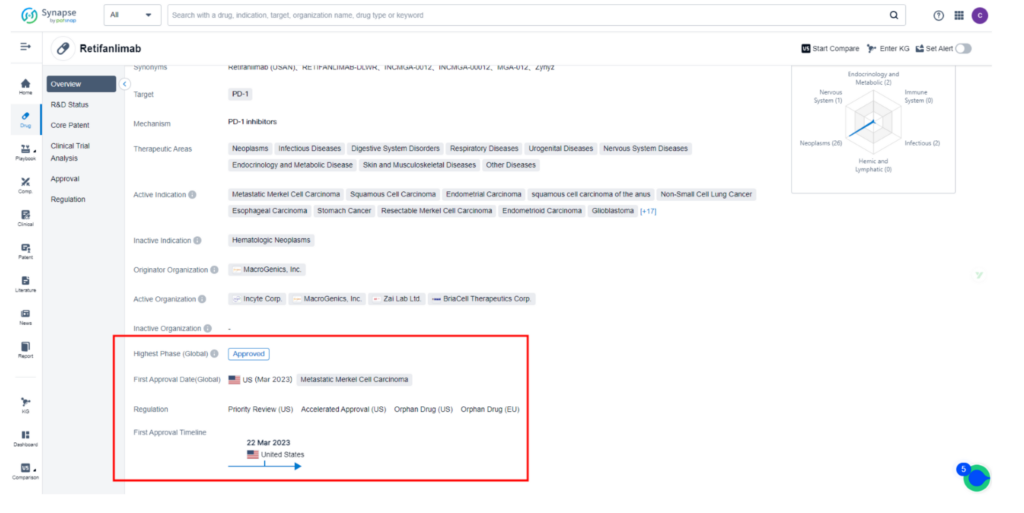
Incyte’s latest development, Zynyz (retifanlimab-dlwr), has received FDA approval for the treatment of adults with metastatic or recurrent locally advanced Merkel cell carcinoma (MCC), targeting programmed death receptor-1 (PD-1).
The drug was granted accelerated approval by the FDA, despite Incyte’s lack of disclosure of an application filing or PDUFA date. The unexpected approval has created excitement in the medical community, opening new doors for MCC patients.
Zynyz: The Eighth Checkpoint Inhibitor to Hit the Market
Zynyz® (Retifanlimab) is a groundbreaking programmed death receptor-1 (PD-1)-blocking antibody that functions by binding to the PD-1 protein on immune T-cells to prevent their interaction with the PD-L1 protein on cancer cells. By doing so, it eliminates the “brakes” on the immune system, enabling it to attack cancer cells more effectively. This innovative approach is revolutionizing Merkel cell carcinoma treatment, offering new hope for patients in need.
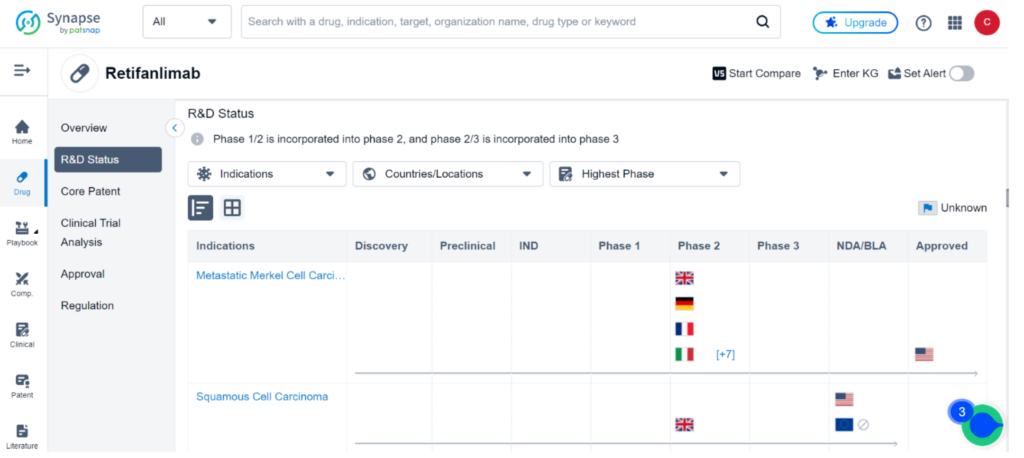
Retifanlimab is currently the subject of 53 clinical trials across the globe, investigating 29 different indications such as Metastatic MCC, Squamous Cell Carcinoma, Endometrial Carcinoma, and Non-Small Cell Lung Cancer.
While the drug has been approved for sale in the United States for Metastatic MCC, other countries are still in clinical Phase 2 development at most. Squamous Cell Carcinoma is currently in the BLA stage in the United States. For comprehensive data on these clinical trials, please refer to Synapse.
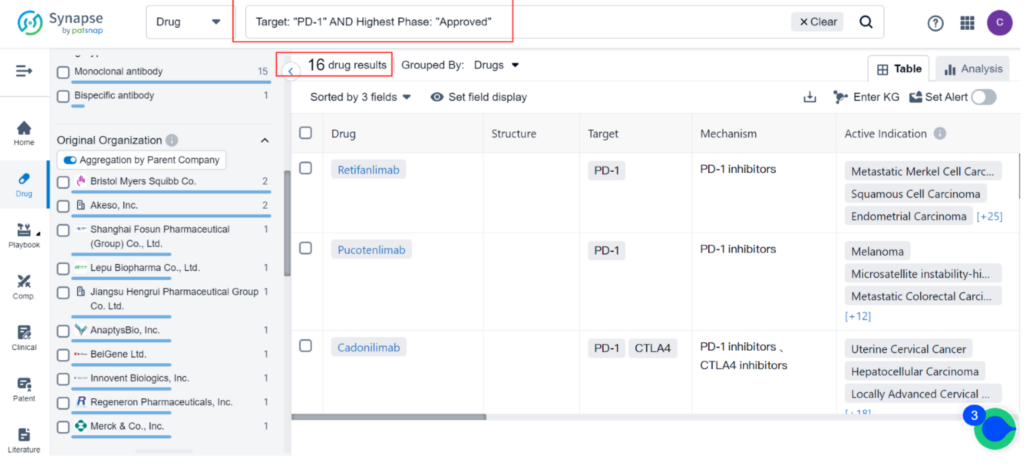
Zynyz is the 16th PD-1 inhibitor to receive approval, with Merkel Cell Carcinoma being the 8th indication. The most common indications for PD-1 inhibitors are Hodgkin’s Lymphoma and Melanoma, followed by Metastatic Merkel Cell Carcinoma, Microsatellite Instability-high Cancer, Uterine Cervical Cancer, Microsatellite Instability Cancer, Cutaneous Squamous Cell Carcinoma, and Esophageal Carcinoma.
Zynyz: Third Drug Indicated for Metastatic MCC
MCC is a rare and aggressive form of skin cancer that usually presents as a solitary, painless, reddish-purple skin nodule on sun-exposed areas such as the head, neck, and arms. Due to its rapid growth and tendency to metastasize, MCC is often associated with poor outcomes, with a five-year overall survival rate of only 14% in patients with distant metastatic disease. Although MCC affects less than one in 100,000 people in the US, its incidence rates are increasing, particularly in those over the age of 65.
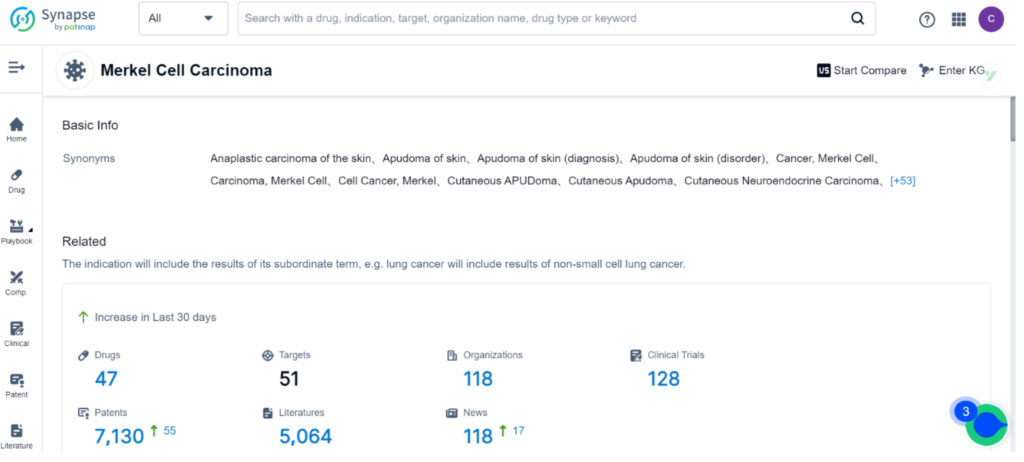
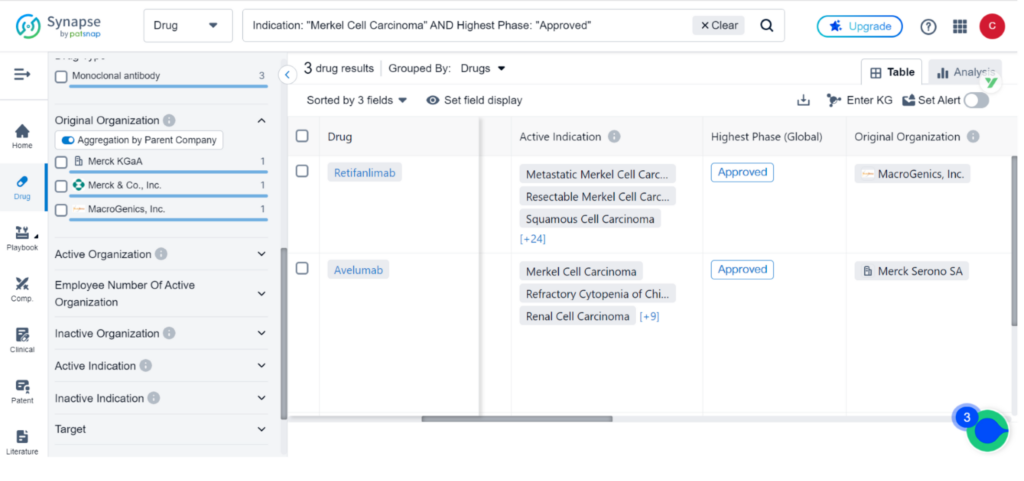
Synapse, the drug intelligence database, contains 47 drugs and 128 clinical trials for the treatment of Merkel Cell Carcinoma. Incyte Corp, Merck & Co., Inc., and Merck KGaA are the top three organizations leading research and development efforts for this indication, with PD-1, PD-L1, and TLR9 being the primary R&D targets. Monoclonal antibody, Small molecule drug, and Fusion protein are the top three types of drug development for MCC.
Zynyz joins the ranks of Avelumab and Pembrolizumab as the third drug approved for the treatment of Merkel cell carcinoma. Avelumab received approval in 2017, while Pembrolizumab followed in 2018.
“Zynyz offers patients and healthcare professionals an additional first-line anti-PD-1 option for patients with metastatic or recurrent locally advanced MCC, which can be a challenging and aggressive disease to treat,” said Hervé Hoppenot, Chief Executive Officer, Incyte. “Incyte is grateful to the investigators and patients around the world who participated in the POD1UM-201 trial. We continue to study the potential of Zynyz in additional tumor types and in combination with other Incyte pipeline compounds.”
To stay informed on drug development and clinical trials in this field, sign up for our freemium product offering, Synapse.
