Key Patent Retrieval Strategies for ADC Drug Innovation
Introduced in 2013, Kadcyla stands at the forefront of targeted therapies for HER2-positive metastatic breast cancer. As a pioneering antibody-drug conjugate (ADC), it offers a tailored treatment option for patients previously treated with trastuzumab and taxane-based regimens, either as monotherapies or in combination.
Using Kadcyla as a case study, we’ll delve into crucial patent retrieval and analysis methodologies that hold the potential to propel drug innovation further.
Jump to . . .
What are ADC Drugs?
Antibody-Drug Conjugates (ADCs) merge the specificity of monoclonal antibodies with the potency of cytotoxic drugs to precisely target cancer cells.
The monoclonal antibody component binds to a specific antigen on tumor cells, ensuring the drug is predominantly delivered to these cells while sparing healthy ones. Upon binding, the ADC-antigen complex is internalized into the tumor cell, where the cytotoxic drug is released due to the cellular environment or enzymatic cleavage.
This released drug then disrupts cellular functions, leading to targeted cell death. The stability of the linker, connecting the drug to the antibody, is crucial; it needs to remain intact in the bloodstream but allow for drug release inside the target cell. The advantage of ADCs is their ability to enhance the therapeutic window by minimizing off-target effects while delivering a potent dose to cancer cells.
What is Kadcyla?
Kadcyla, chemically known as ado-trastuzumab emtansine, is an ADC. This means it’s a fusion of two main components:
- Trastuzumab: This is a monoclonal antibody that specifically targets the HER2 receptor, which is overexpressed in certain types of breast cancers. Monoclonal antibodies, as you may know, are produced to bind to specific molecular targets, providing selectivity.
- DM1 (Emtansine): This is a cytotoxic agent, an organic small molecule that interferes with the microtubule assembly of cells, leading to cell death. It’s highly potent, so if it were given on its own, it could lead to severe side effects. But when linked to trastuzumab, its effects are largely confined to the HER2-positive cancer cells.
The two components are linked via a stable thioether linker, ensuring the cytotoxic payload isn’t prematurely released before reaching its intended target. Once Kadcyla binds to the HER2 receptor on a cancer cell, the entire complex is internalized via endocytosis. Inside the cell, the linker is cleaved, and DM1 is released, inducing cytotoxic effects.
Patent Retrieval Steps
In this section, we’ll dive into the patent retrial process for Kadcyla. To get started, register for a free account with Patsnap’s Bio Sequence Database. This comprehensive, AI-powered database provides you with 24/7 access to millions of sequences from patents, degenerate sequences, and non-patent literature sequences.
Once you create your account, head to the homepage and type “Kadcyla trastuzumab” in the drug/gene index search bar to retrieve the sequence details of this antibody.
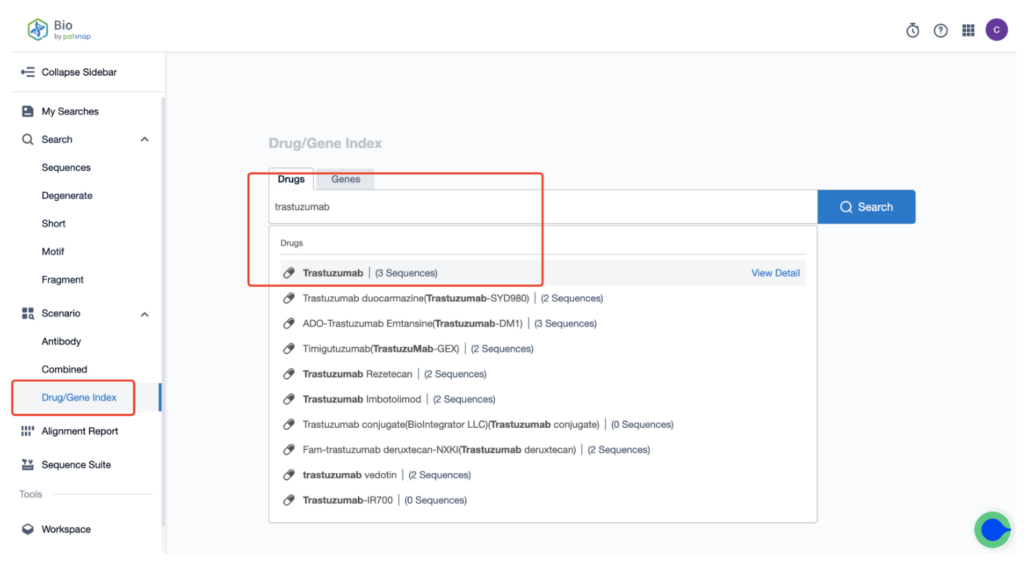
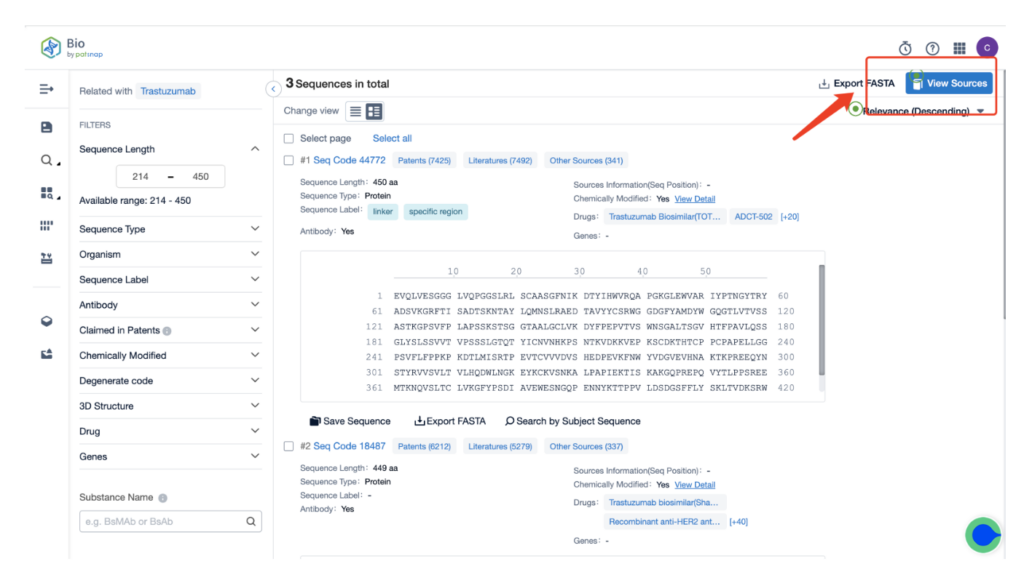
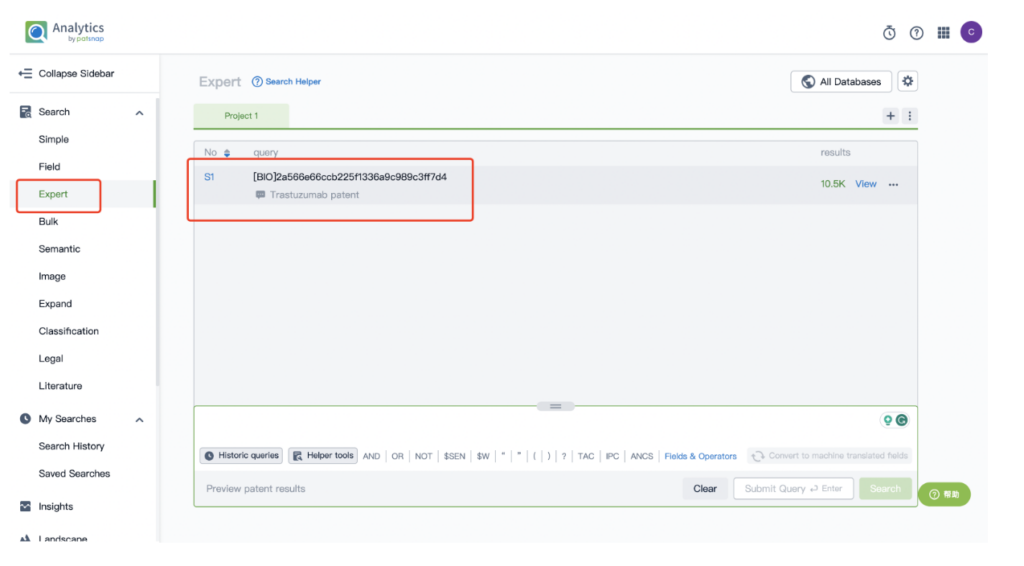
Next, select “View in Analytics” located at the top right corner to enter the patent database.
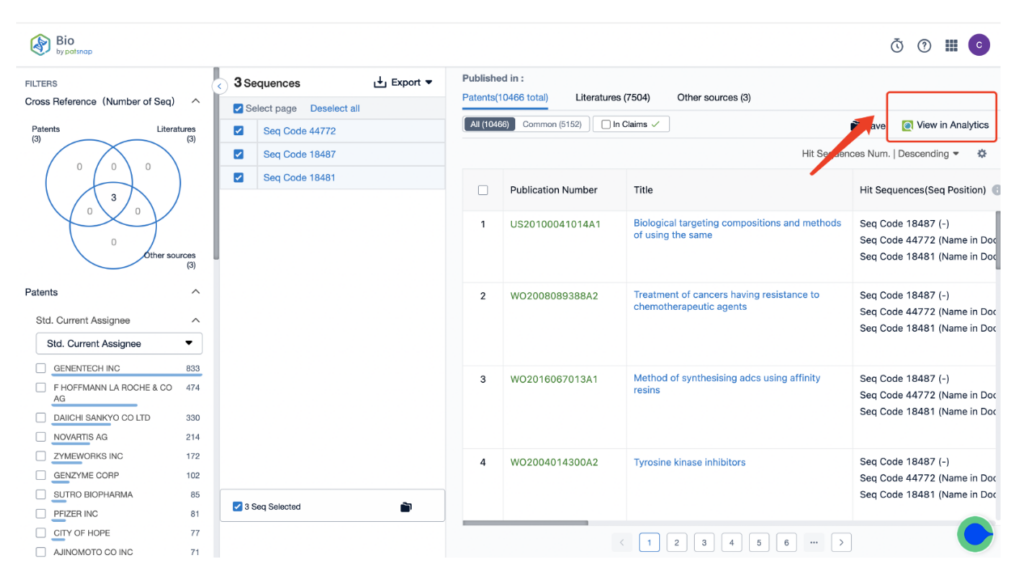
From there, input a search for the monoclonal antibody Trastuzumab, associated with the ADC drug Kadcyla, in the search field.
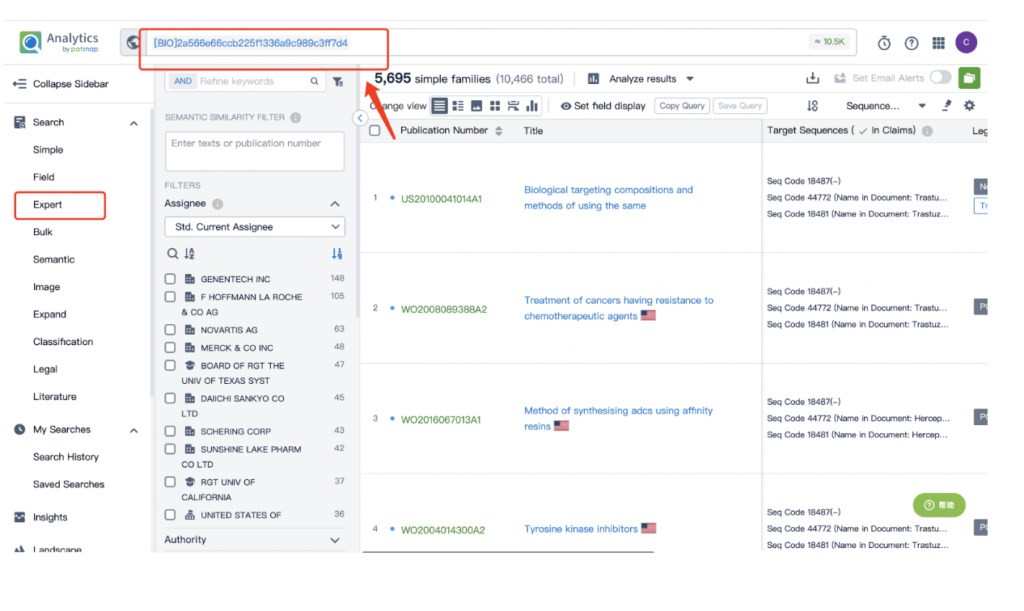
Remember to store this query in the expert search library for future reference.
Then navigate to the Chemical database. Here, you’ll want to search for patent details related to Kadcyla’s MCC linker and its potent cytotoxic agent, DM1.
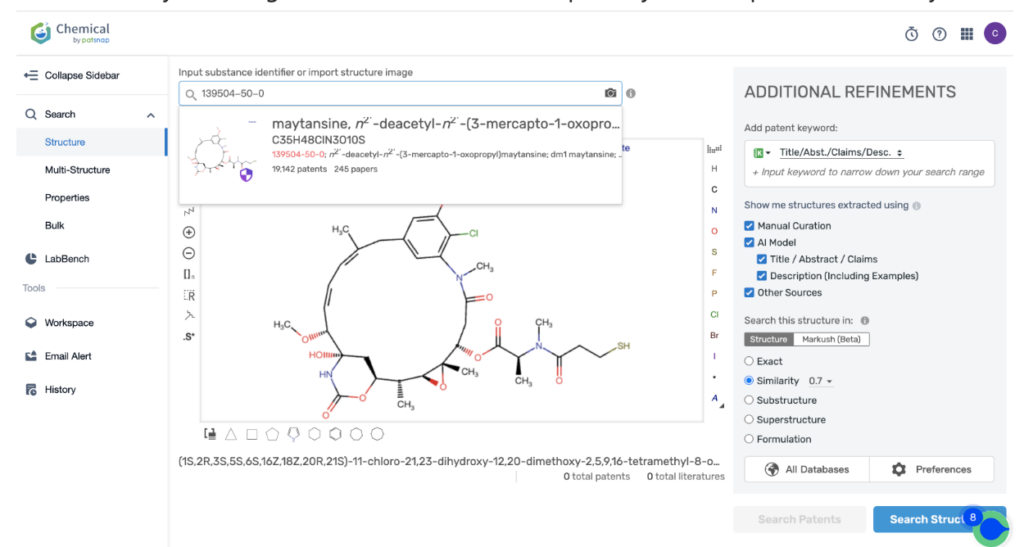
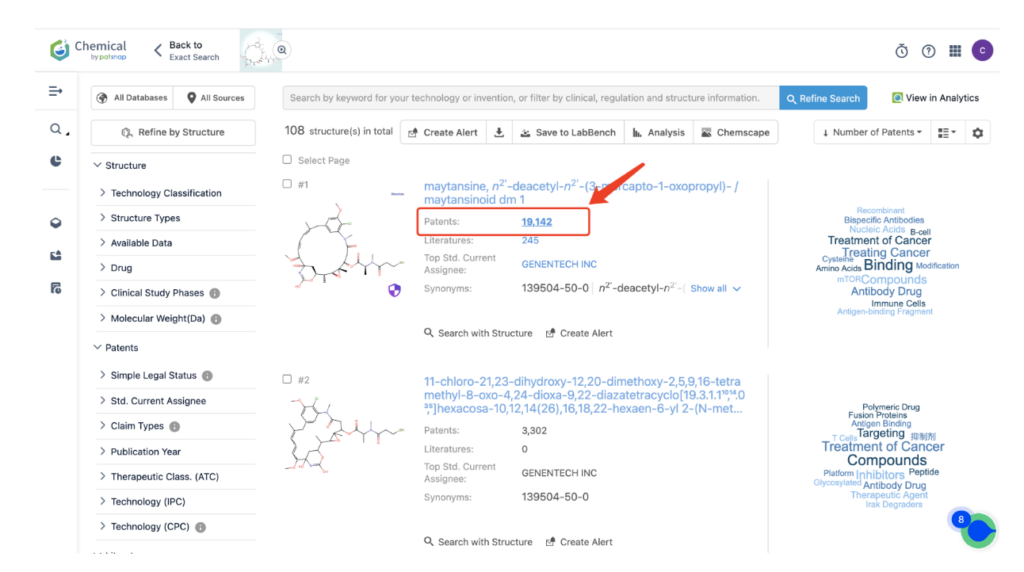
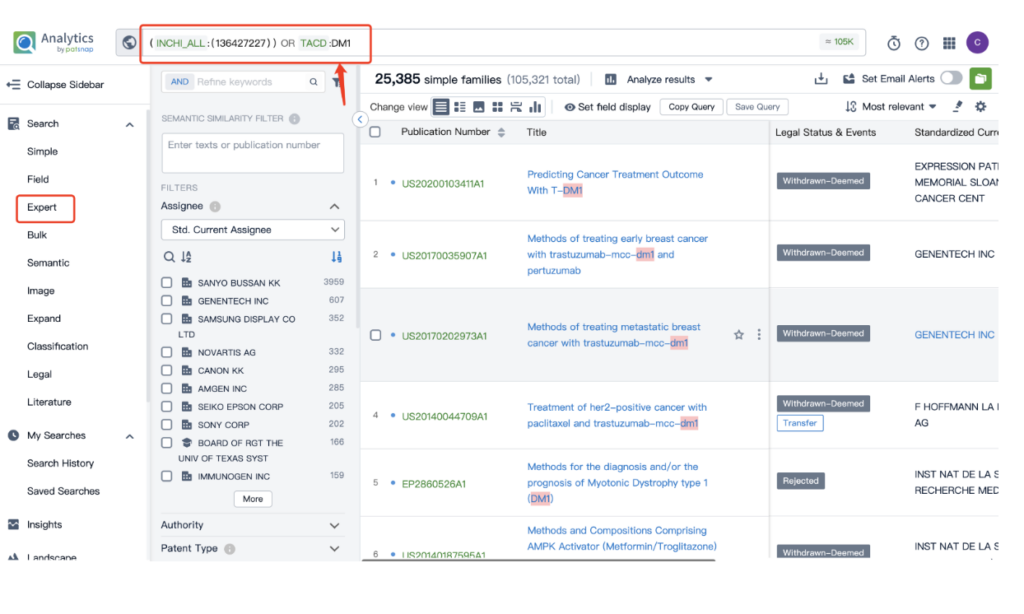
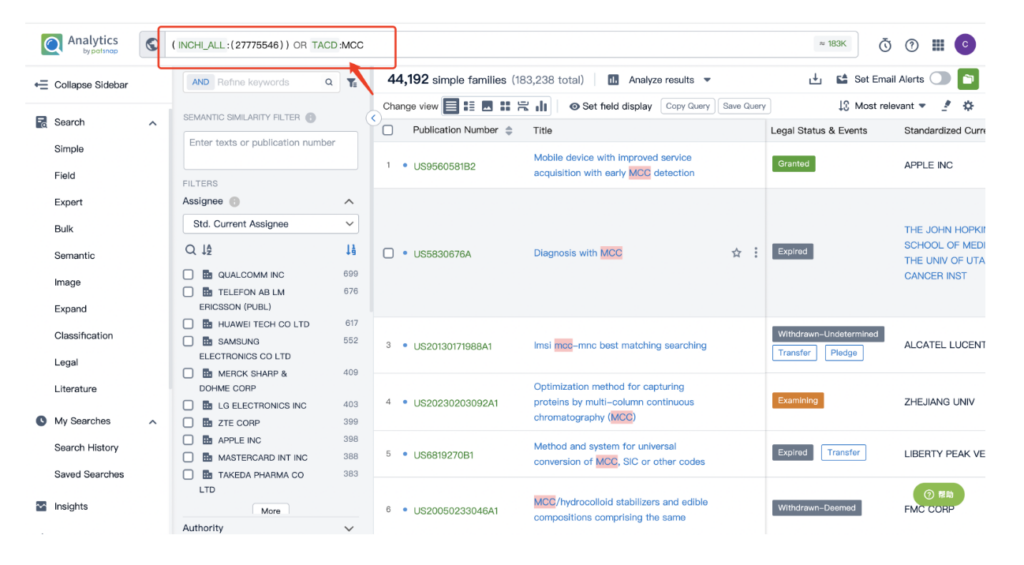
Once retrieved, store each search separately in the expert search library for easy reference later.
By performing an ‘AND’ operation in the expert search library and combining these three retrieval formulas, you can quickly retrieve relevant patent results for Kadcyla (or whatever drug you’re researching).
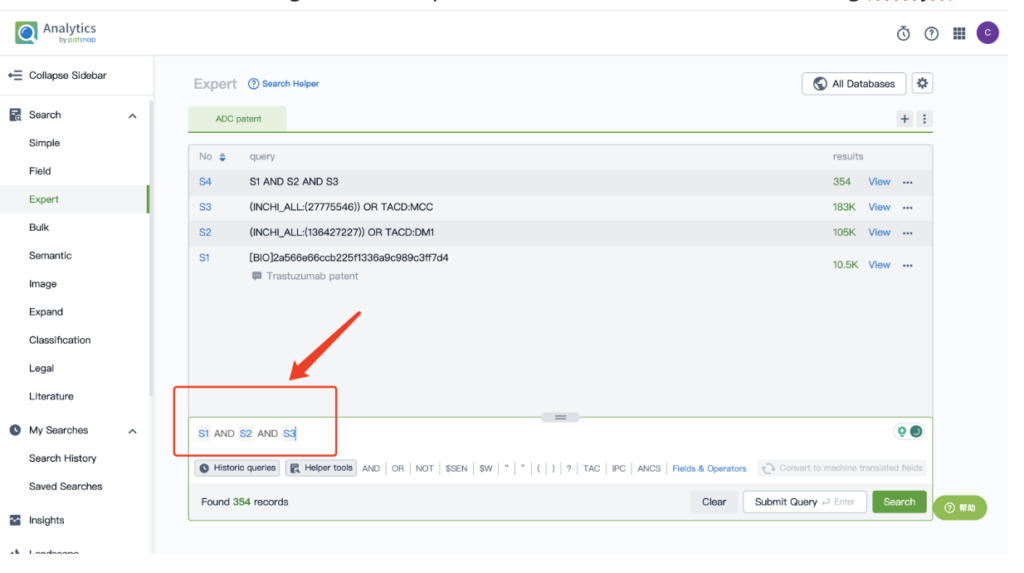
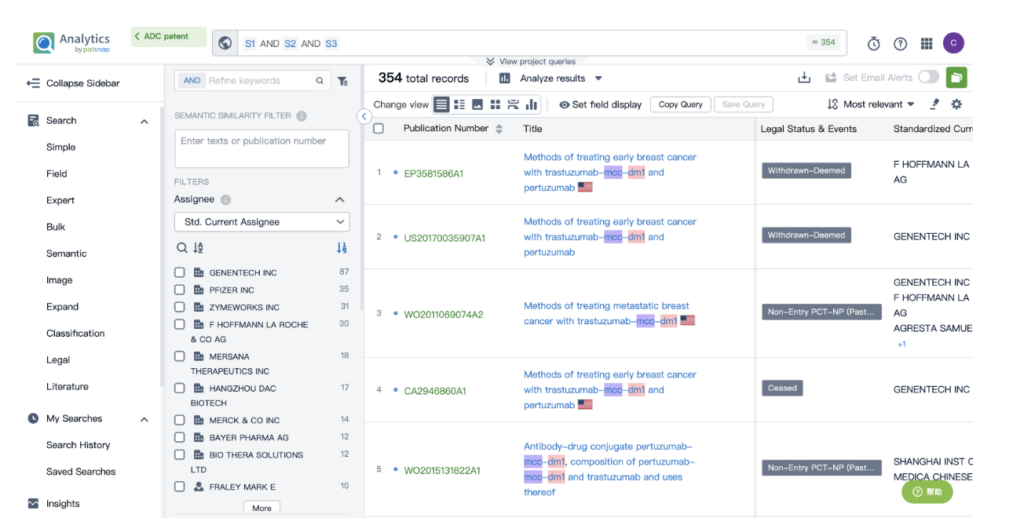
This article offers a glimpse into the powerful insights offered by Patsnap’s AI-driven database. For a deeper dive into biosequence search and analysis, sign up for Patsnap Bio here.

