Life Science Snapshot: Advancements in Breast Cancer Treatment
Breast cancer affects approximately 12.5% of women, making it the most common cancer in the world. One out of every three cases will become metastatic. In this article, we’ll uncover treatment advancements.
Breast cancer is the most common cancer worldwide, with approximately one in eight women affected in their lifetime. It also impacts men, although to a lesser degree. There are many types of breast cancer, all of which are determined by the cells in the breast that become cancerous. It occurs when cells in the breast begin to grow abnormally which causes the affected cells to rapidly divide. As a result, a lump or mass forms, and in more advanced cases, the cancer spreads to lymph nodes and other parts of the body.
Numerous processes are used to diagnose breast cancer. These include: a breast ultrasound, mammogram, MRI, or biopsy (which requires a sample of tissue or fluid from the breast). Once diagnosed, the patient goes through a staging process to determine whether the cancer cells have spread or are starting to spread. This also allows medical professionals to outline the best treatment options.
In this article, we’ll explore advancements in breast cancer treatment.
This article was made using Synapse. Keep track of advancements in drug developments and clinical trials with Synapse.
Advancements in HER2-low Breast Cancer Treatment
Targeted therapies are emerging for HER2-low breast cancer treatment. This specific type of cancer produces tumors with low levels of the HER2 protein on the tumor surfaces. To put this in context, these tumors account for 50 to 60 percent of all types of breast cancer.
Enhertu is an antibody-drug conjugate/monoclonal antibody that was approved by the FDA in August of 2022 for the treatment of patients with unresectable or metastatic HER2-low breast cancer. This approval marked the first actual targeted therapy for patients with HER2-low breast cancer and is hopefully the start of a new treatment trend.
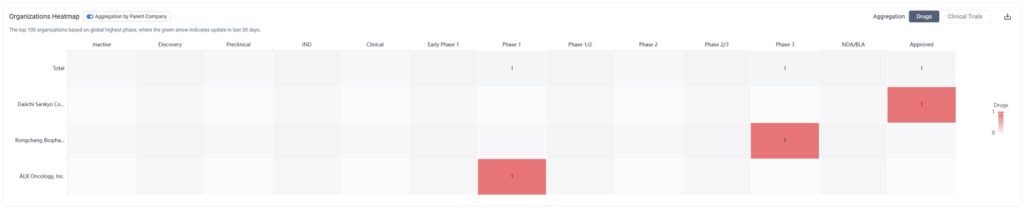
By zooming into the image above, we can see that although there is only one drug currently approved for HER2-low breast cancer, two others are in clinical trial phases.
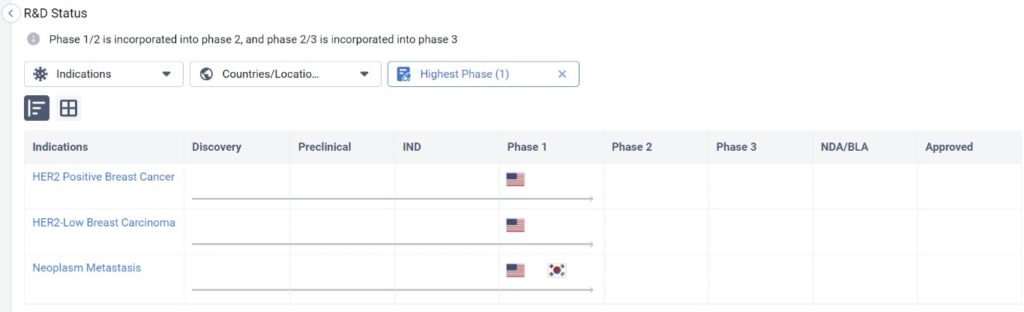
One of these drugs is ALX Oncology Inc’s Evorpacept, which is in Phase I for HER2 Positive Breast Cancer, HER2-Low Breast Cancer Carcinoma, and Neoplasm Metastasis as well. Simultaneously, it’s in Phase III for the treatment of stomach cancer, and is also being tested for other cancer indications.
Drugs in this phase are usually studied for several years, although that timeframe is shortening thanks to advancements in technology (read this eBook for more information). However, only 25 to 30 percent of these drugs move onto the drug application stage.
In the years to come, we expect to see additional treatment options emerge people breast cancer treatment.
mRNA Vaccine: An Effective Breast Cancer Treatment Method?
mRNA tech works by targeting the genetic makeup of virus cells at their core, which enables them to reprogram and produce antiviral antigens or proteins. However, it’s not as effective in its natural form because the body recognizes it as a foreign invader, causing the body to attack and destroy it.
This is where things get interesting. When modified (in lipid form), mRNA tech becomes “invisible” to the immune system. As a result, it can rally the necessary cells to help defeat viruses and other diseases.
This modification is what enabled Pfizer-BioNTech and Moderna scientists to deliver a COVID vaccine that’s 90% effective — up from a mere 48% without it.
This begs the question: could COVID vaccine tech crack cancer treatment?
It’s plausible… and we aren’t the only ones asking this question.
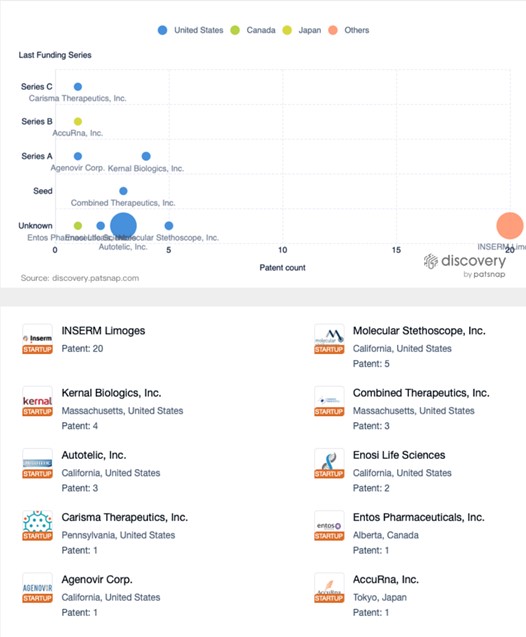
The graph below illustrates startup companies in the mRNA therapeutics space, with Inserm Limoges emerging as a top patent filer. In the future we may see companies like BioNTech, which are actively researching mRNA tech for cancer treatment, partnering with some of the companies on this list.
Meanwhile, other companies are thinking outside of the box. Instead of using mRNA vaccine treatment specifically, they’re focusing on the learnings extracted from the COVID vaccine innovation process.
Take RNA Nanotherapeutics as an example. It’s a startup founded by Xiaoting Zhang that focuses on the treatment of breast cancer. The company uses multifunctional RNA nanoparticles to target breast cancer cells and block the production of MED1 proteins — which are a co-driver of the disease. And its efforts are paying off — recently RNA Nanotherapeutics received a Small Business Technology Transfer Phase I grant from the National Cancer Institute.
With more investments funneling into vaccine treatments to not only treat breast cancer but also cancer in general, we can expect cancer treatment options to move by leaps and bounds in the future.
Furthermore, according to a recent report by Allied Market Research analysts, the global cancer vaccine market was valued at about $4.8 billion in 2019, and it’s expected to reach a $7.3 billion dollar valuation by 2027. We all know money talks and based on this valuation we can expect to see an uptick in both innovation, and new and emerging players.
Conclusion
As we’ve outlined in this article, the breast cancer sector is ripe with research and innovation. In the coming years, we expect to see more viable treatment options materializing that can save, extend, and improve lives.
If you’re interested in learning more about this space, or keeping track of drug development and clinical trials, sign up for Synapse, our free product offering.
Author Bio

Nicholas Bevilacqua is the Product Marketing Manager For the Life Sciences Platforms at PatSnap. Nicholas holds an Honors Bachelors of Life Sciences from York University and an MBA in Strategic Marketing from the DeGroote School of Business at McMaster University. In his spare time, he enjoys cooking and outdoor activities.