Life Science Snapshot: Multiple Sclerosis Detection & Treatment Advancements
Multiple Sclerosis (MS) is a chronic disease that impacts the central nervous system. Although there is no cure for MS, advancements in detection and treatment are on the horizon. In this article, we’ll explore a few promising clinical trials.
We curated this article using insights from our Synapse platform. Keep track of advancements in drug developments and clinical trials when you register for Synapse.
Multiple Sclerosis Overview
Multiple Sclerosis, also known as MS, is a chronic disease that impacts the central nervous system. It occurs when the immune system attacks the nerve fibers and myelin sheathing in the brain and spinal cord. MS is unpredictable and affects every person differently. Common symptoms include numbness or weakness in limbs, electric shock sensations, tremors, and vision problems.
Currently, there is no cure for MS, but research advancements are underway. In this article, we’ll uncover a few advancements in detection and treatment.
MS Treatment Advancements
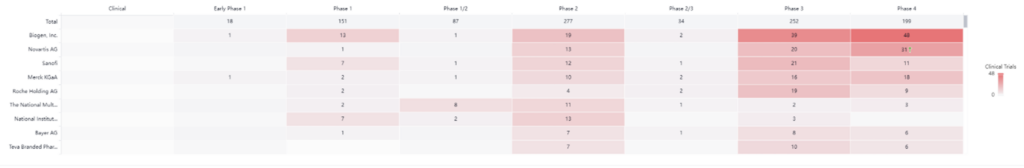
As the graph above illustrates, there are many clinical trials happening in the MS space. Companies such as Biogen (with 48 active phase IIII trials) and Novartis (with 31 active phase IIII trials) are leading the way. However, this isn’t stopping smaller players from making strides and advancements, which we’ll explore in more detail in the next section.
One drug candidate worth mentioning is Clene Nanomedicine’s gold nanocrystal suspension, CNM-Au8. This drug candidate is currently being tested to treat MS, Parkinson’s, and Amyotrophic Lateral Sclerosis. The graph below indicates the regions these clinical trials are taking place in, and automatically updates to track progress and advancements for the drug.
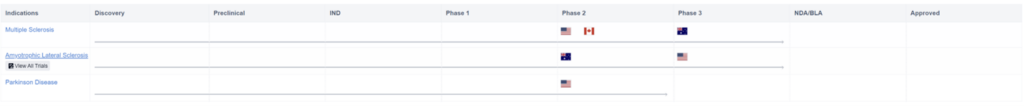
Additionally, the company also reported positive topline results from its phase II VISIONARY-MS trial. This trial measures the safety and efficacy of CNM-Au8 on patients that are suffering from relapsing-remitting multiple sclerosis (RRMS). The participants were split into two groups. Group one was given 15mg or 30 mg daily dosages as adjunctive therapy to available DMTs. Group two was given a placebo.
After 48 weeks, the trial was stopped early (in February of 2022) due to challenges related to COVID-19. That said, CNM-Au8 showed improvements across multiple paraclinical biomarkers and passed secondary endpoints including a 25-foot timed walk and a finger dexterity test, in comparison the patients on placebo worsened over the same period. Thus far, Clene feels hopeful about these results and looks forward to initiating a phase III study.
Advancements in Detecting MS
When it comes to any indication it’s important to understand the severity of the disease in order to assess the proper treatment needed, and MS is no different. A study done by the medicine University of Vienna shows that for the first time ever that the retina can be used as a prognostic marker for MS.
The study revealed that thinning in the retinal layer because of MS relapse can predict the severity of a future relapse in a patient and in turn the probability of disability. Thus, study and analysis confirmed the loss of approximately 5 µm of retinal layer thickness after optic neuritis can lead to a doubling of the risk of permanent disability in an individual’s next relapse. These results suggest more aggressive treatment should be indicated where there is a significant thinning in the retinal layer.
Conclusion
Advancements in the treatment and detection of MS are promising. As research continues to progress, we expect to see new therapies hit the market aimed at improving quality of life by reducing the frequency and severity of MS symptoms. If you’re interested in learning more about this space, or keeping track of drug development and clinical trials, try Synapse for free.
Author Bio

Nicholas Bevilacqua is the Product Marketing Manager For the Life Sciences Platforms at PatSnap. Nicholas holds an Honors Bachelors of Life Sciences from York University and an MBA in Strategic Marketing from the DeGroote School of Business at McMaster University. In his spare time, he enjoys cooking and outdoor activities.
