Life Science News: Spinal Muscular Atrophy R&D Trends
Although there is no cure for Spinal Muscular Atrophy, new treatments are emerging. In this article, we’ll explore the research, news, and clinical trials shaping the future of SMA.
Spinal Muscular Atrophy (SMA) affects one in 8,000 people worldwide and is a genetic disease that affects the central nervous and peripheral nervous systems, along with muscle movement.
It’s progressive, meaning it gets worse as time goes on. Left untreated, SMA sufferers will develop issues walking, talking, breathing, and moving. This leads to muscular atrophy and overall weakness.
Currently, there is no cure for SMA, but new treatments (such as Spinraza) are emerging. In this article, we’ll explore advancements in treatments and indications.
SMA Research
Recent news shows advancements in research in the SMA space. For example, researchers at Cold Spring Harbor partnered with Universidad de Buenos Aires and found pairing Spinraza (nusinersen) with a second treatment, valproic acid (VPA), boosts therapeutic effects.
Approved in 2016, Spinraza is the first FDA treatment for SMA. It works by targeting the SMN2 gene and enabling it to better support the motor neurons responsible for breathing, eating, speaking, and movement.
One way to improve the impact of a drug is to simply increase the dosage, but experts said increasing the amount of Spinraza could lead to toxic side effects. However, pairing it with VPA may increase the clinical effects. VPA will allow Spinraza to increase output of the SMN protein, a protein that SMA patients do not have enough of. Currently, clinical trials using valproic acid are underway, as indicated in the graph below.
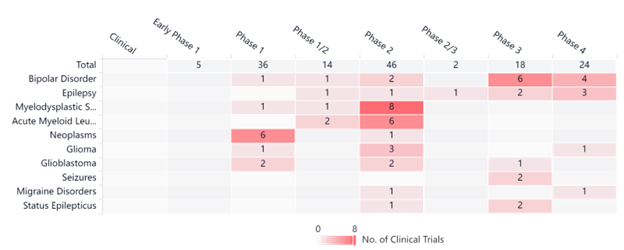
Interestingly, phase 4 VPA trials happening now include bipolar disorder, epilepsy, and glioblastoma — all of which are spinal or brain disorders and neurodegenerative like SMA.
SMA News
In addition to new therapies in clinical trials for adolescents and adults, infant treatments are also being explored. In May of 2022, Roche received approval from the FDA for the use of Evrysdi (risdiplam) infants younger than two months.
Initial approvals for Evrysdi were granted in August of 2020. It was originally used to treat adults and children with SMA, so this expansion represents a massive leap in infant treatment. Based on the RAINBOWFISH clinical trial for newborns, Evrysdi showcased that most pre symptomatic babies who received the drug were able to hit milestones of sitting and standing. Furthermore, half of the users were able to walk following a year of treatment. To put this in perspective, many children with SMA are never able to sit up on their own, stand, or walk.
The graph below, taken from PatSnap’s Synapse Platform, showcases various Evrysdi clinical trials currently taking place. We expect to see more developments in the future.
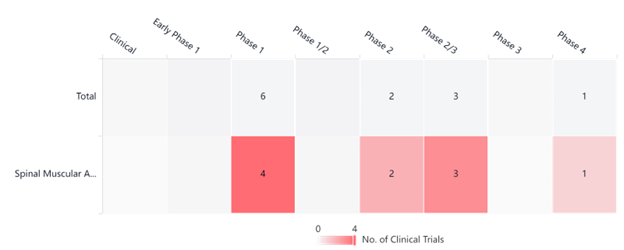
SMA Clinical Trials
Both drugs mentioned above target the SMN2 motor neuron gene that encodes the survival motor neuron protein (SMN). The Synapse graph below illustrates all clinical trials being done on targeted genes in the SMA space. Although SMN2 is the gene that is most targeted, there are advancements in clinical trials for various other genes with some clinical trials progressing to later phases.
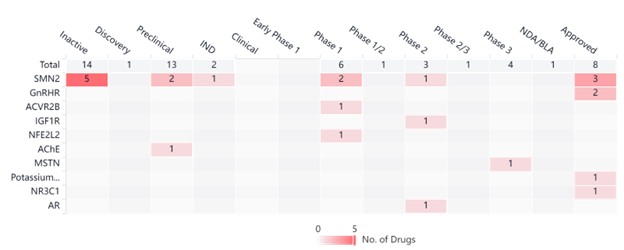
Conclusion
Although there is no cure for SMA, new therapeutic advancements are underway. If you’re interested in learning more about this space, or keeping track of drug development and clinical trials, sign up for Synapse, our freemium product offering.
Author Bio

Nicholas Bevilacqua is the Product Marketing Manager For the Life Sciences Platforms at PatSnap. Nicholas holds an Honors Bachelors of Life Sciences from York University and an MBA in Strategic Marketing form the DeGroote School of Business at McMaster University. In his spare time, he enjoys cooking and outdoor activities.
