Merck’s Daily PCSK9 Pill: Hypercholesterolemia Patients
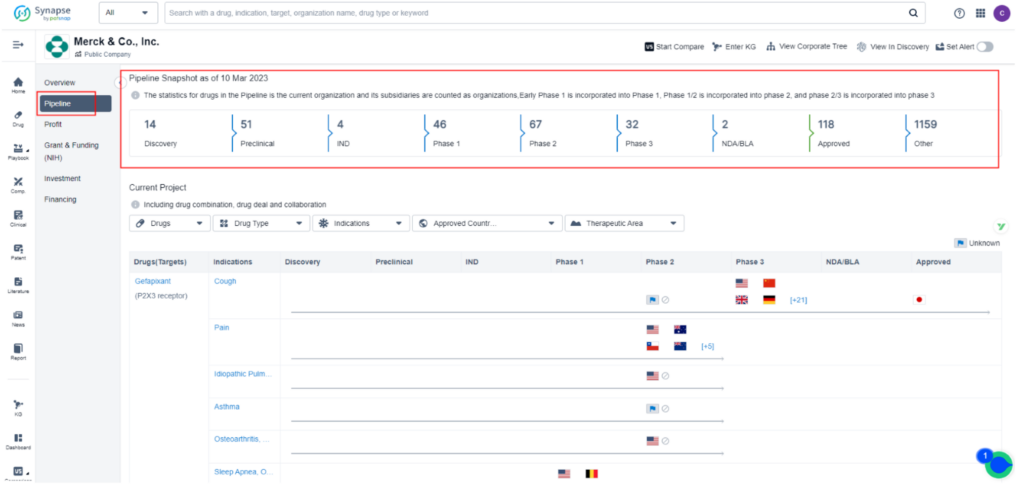
Merck & Co is pushing forward with its Phase 3 program to test the efficacy of MK-0616, an oral cholesterol-lowering drug that has shown promise in mid-stage trials by matching the effects of approved injectable competitors.
This development is particularly significant in the fight against hypercholesterolemia, a lipid disorder characterized by high levels of LDL or “bad” cholesterol that can lead to atherosclerosis, a condition in which fat accumulates in the arteries and increases the risk of heart attack and stroke.
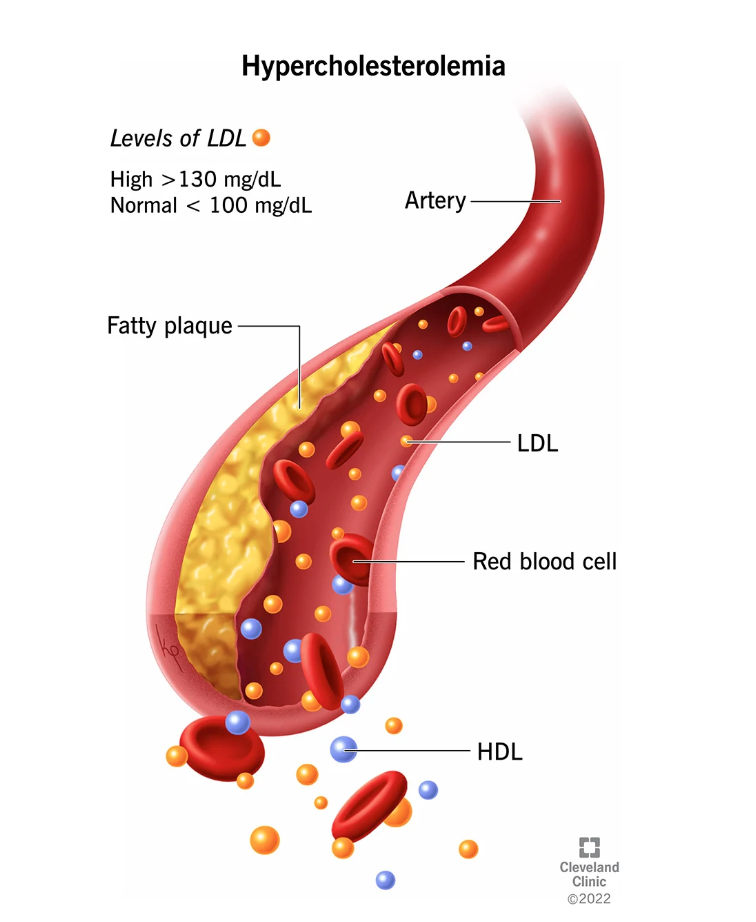
As cardiovascular disease remains the leading cause of death worldwide, the success of Merck’s program could have a profound impact on patients and healthcare systems.
In this article, we’ll examine the data behind this new drug.
Overview of Hypercholesterolemia Medications
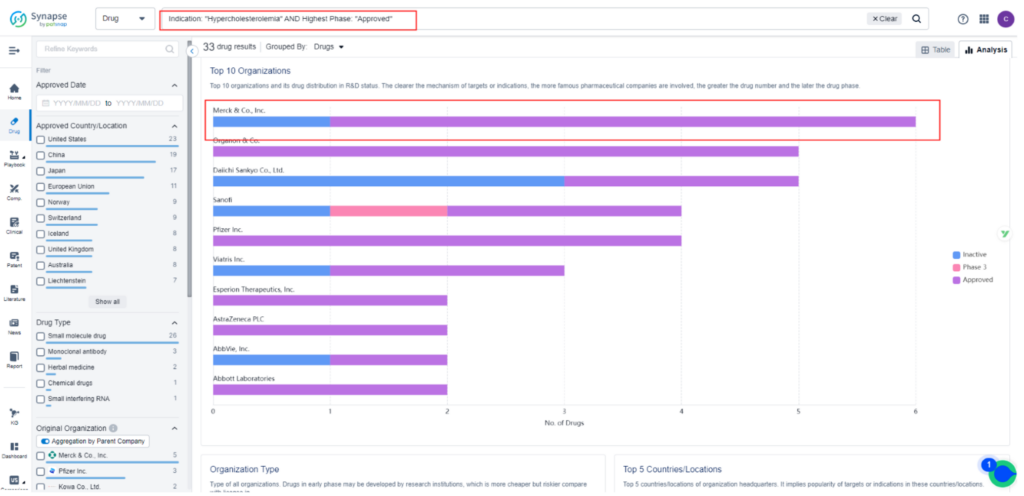
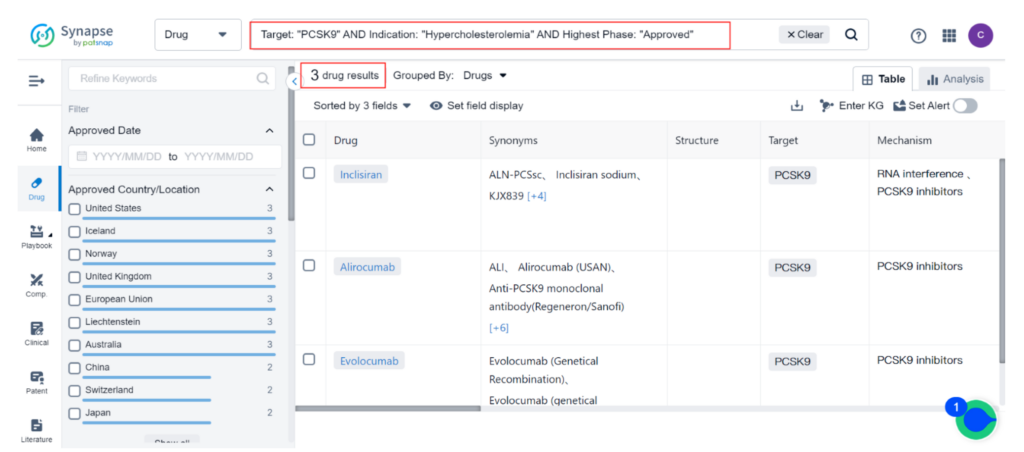
Currently, there are 33 approved treatments for Hypercholesterolemia, the majority of which are small-molecule drugs targeting HMG-CoA reductase inhibitors. Only three approved drugs target PCSK9 inhibitors, and all of them are injectables. Among the injectable PCSK9 inhibitors, Amgen’s Repatha and Regeneron’s Praluent have been available in the U.S. for years.
Despite their potent effects on cholesterol levels and modest cardio-protective benefits, they have struggled to gain popularity among physicians and patients. Novartis’ Leqvio was more recently approved as a third option.
MK-0616: Revolutionizing Hypercholesterolemia Treatment?
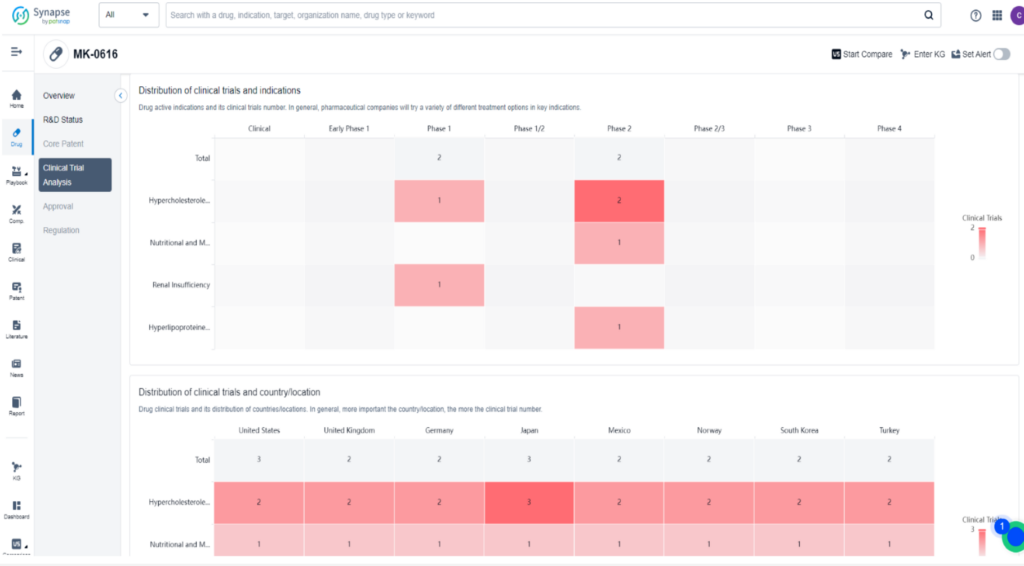
MK-0616 is a novel oral PCSK9 inhibitor developed by Merck to lower low-density lipoprotein (LDL) cholesterol, which is a major contributor to cardiovascular disease. This macrocyclic peptide drug works by binding to PCSK9 and inhibiting its interaction with LDL receptors. In a Phase 2b clinical trial, MK-0616 was tested on 381 adults with high cholesterol and varying heart disease risk at four different doses. The results showed that the drug lowered LDL cholesterol levels by 40% to 60% compared to a placebo in just eight weeks without any significant side effects.
This promising data has led Merck to launch broader testing, including lipid-lowering studies, a heart outcomes trial, and supportive studies in different patient subgroups. The goal is to establish MK-0616 as the preferred add-on to statins and potentially revolutionize the treatment of hypercholesterolemia.
Merck’s PCSK9 Pill: Targeting Success
Merck’s success with MK-0616 also highlights the company’s expertise in small molecule drug discovery, as some competitors have ended their own oral PCSK9 programs. For example, Novo Nordisk terminated the development of its rival oral PCSK9 drug in late 2020.
Merck has a longstanding history of developing treatments for cardiovascular disease, dating back more than 60 years. The company’s commitment to understanding and addressing cardiovascular-related disorders remains strong, as cardiovascular disease remains one of the most significant health challenges of the 21st century. Globally, approximately 18 million people die each year due to cardiovascular disease, and in the United States, one person dies every 36 seconds from the condition.
“Our interest is to broaden and democratize PCSK9 inhibition as a tool for patients obtaining their LDL cholesterol [reduction goals],” stated Eliav Barr, the Chief Medical Officer of Merck Research Laboratories. Merck intends to conduct the cardiovascular outcomes trial, which is crucial for the marketing of a heart drug, simultaneously with the lipid studies, which may provide faster answers despite the high cost involved.
If you’re interested in learning more about this space or keeping track of drug development and clinical trials, sign up for Synapse, our freemium product offering.
